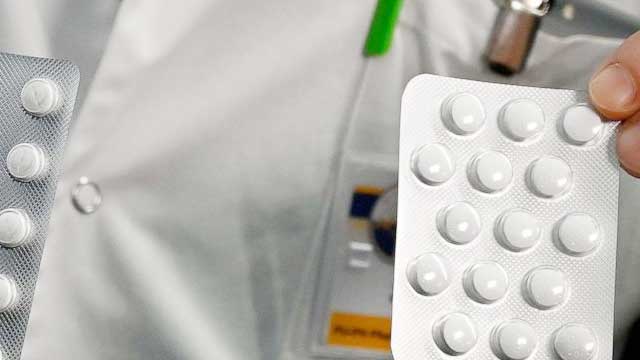
The U.S. Food and Drug Administration has issued a limited emergency-use authorization for two antimalarial drugs to treat those infected with the novel coronavirus.
In a statement released Sunday night, the U.S. Department of Health and Human Services announced it had received 30 million doses of hydroxychloroquine sulfate and one million doses of hloroquine phosphate donated to a national stockpile of potentially life-saving pharmaceuticals and medical supplies. Hydroxychloroquine and chloroquine, which are oral prescription drugs used primarily to prevent and treat malaria, are both being investigated as potential therapeutics for COVID-19.
The statement noted that the FDA had issued an emergency-use authorization to allow both donated drugs "to be distributed and prescribed by doctors to hospitalized teen and adult patients with COVID-19, as appropriate, when a clinical trial is not available or feasible."
Federal agencies, such as the National Institutes of Health and the Biomedical Advanced Research and Development Authority, are working together to plan clinical trials.-ABC News